Expertise
Move at the Speed of Experience™
Extensive Real-World Expertise and Proficiency
Collectively, RCP brings more than 125 years in rare disease and 30 years in gene therapy of combined global commercialization experience having created new markets and launched 16 products across 17 therapeutic areas.
Our Private Markets practice is uniquely positioned to service our Private Equity and Venture Capital owned clients. Optimized commercial readiness is a path to quickly unlock an organizations potential, drive Time to Value and ultimately have a positive impact to EBITDA.
Our value comes from the depth and breadth of real-world experience.
Select Areas of Expertise
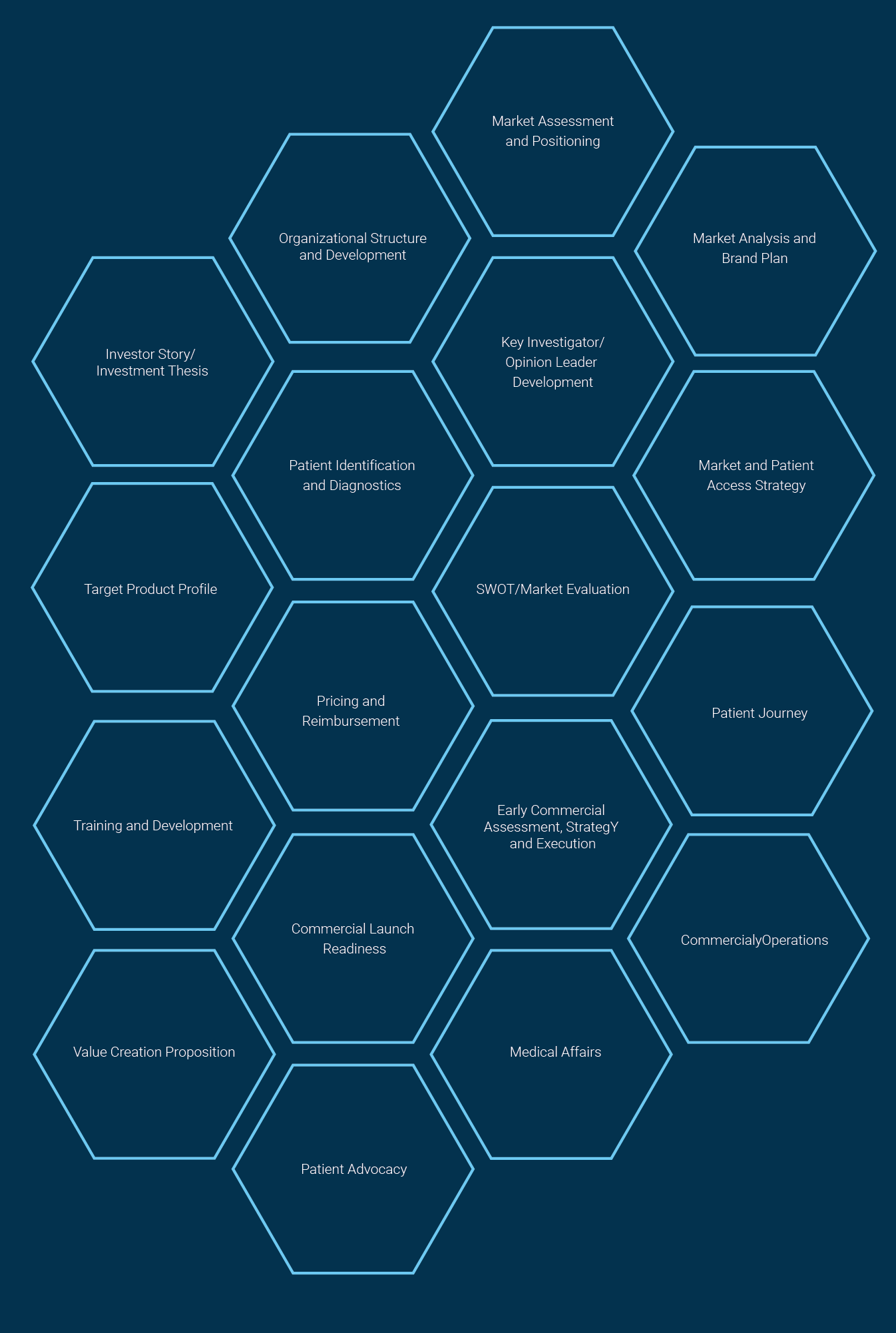
Value Creation Opportunities
Commercial Fundamentals
-
Commercial strategy and marketing
-
Market assessment and positioning
-
Shared medical services
-
Investor story/slide deck
-
Investigator/ Key Opinion Leader (KOL) identification and development
-
Patient identification and diagnostics
-
Patient journey
-
Diagnostic landscape analysis
-
Disease awareness and branded campaigns
-
Advisory boards, roundtable meetings and symposia
-
Team development/optimization
Medical Device Commercialization
How RCP can Help Bring Clarity to Your MedTech Venture
-
Uncover the Hidden Barriers to successful commercialization of new medical device technologies
-
Develop True Product Viability and Market Value with market feedback, product positioning, and pricing strategies
-
Establish a market presence through clinical evidence, regulatory and reimbursement guidance, and development of appropriate distribution channels